
The average atomic mass of titanium will thus beĪvg. For chemistry students and teachers: The tabular chart on the right is arranged by Atomic mass (weight). The decimal abundances for these five isotopes will be: Simply put, each isotope i will contribute to the average atomic mass of the element in proportion to its decimal abundance, which is simply the percent abundance divided by 100.Ī v e r a g e a t o m i c m a s s = ∑ i m a i × a b u n d a n c e o f i which may also be called standard atomic weight or average atomic mass. What is the average atomic mass of titanium 4) Why is the mass in amu of a carbon-12 atom reported as 12.011 in the periodic table of the elements 5) Naturally occurring chlorine that is put in pools is 75.53 percent 35Cl (mass 34.969 amu) and 24.47 percent 37Cl (mass 36.966 amu). Now, the average atomic mass of titanium is calculated by taking the weighted average of the atomic masses of its stable isotopes. Element: Titanium Symbol: Ti Atomic Mass: 47.867 of Atoms: 1 Mass Percent: 100.000. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine. When the problem doesn't provide you with the actual atomic mass of an isotope, m a , you can use its mass number, A, as an approximation of its atomic mass. hexagonal close-packed (hcp) Titanium is a chemical element with the symbol Ti and atomic number 22. The average atomic mass of titanium will thus beĪvg.


Simply put, each isotope i will contribute to the average atomic mass of the element in proportion to its decimal abundance, which is simply the percent abundance divided by 100.Ī v e r a g e a t o m i c m a s s = ∑ i m a i × a b u n d a n c e o f i On another planet, the isotopes of titanium have the given natural abundances. Titanium (atomic weight 47.87, atomic number 22) is named after the Titans, who were the first sons of the Earth Goddess Gaia in Greek mythology. Bromine has two isotopes 79 Br and 81 Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69 and 49.31) were determined in earlier experiments.
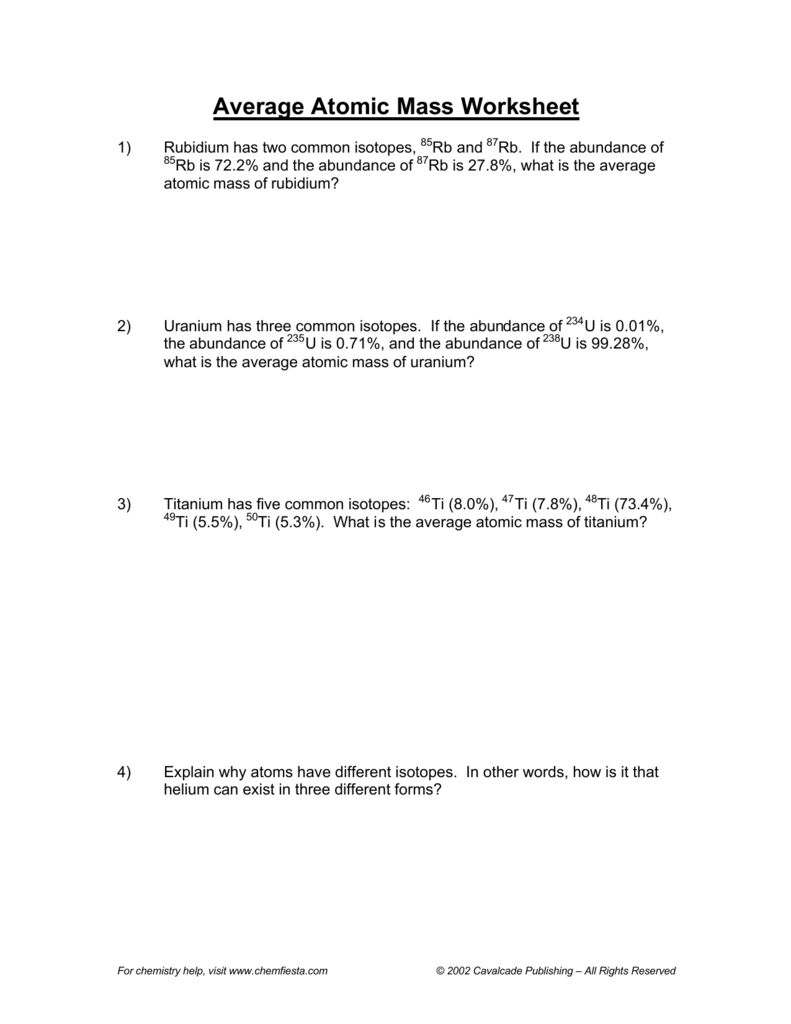
Average atomic masses listed by IUPAC are based on a study of experimental results. Now, the average atomic mass of titanium is calculated by taking the weighted average of the atomic masses of its stable isotopes. Calculate the average atomic mass of this element. (Isotope,Abundance,Mass )(u)( 46Ti, 71.100, 45.95263 48Ti 19.000 47.94795 50Ti 9.900 49.94479 What is the average atomic mass of titanium on that planet On another planet, the isotopes of titanium have the given natural abundances. When the problem doesn't provide you with the actual atomic mass of an isotope, m a , you can use its mass number, A, as an approximation of its atomic mass.


 0 kommentar(er)
0 kommentar(er)
